Abstract
Introduction: There are still limited data on the efficacy of the third-dose (booster) coronavirus disease 2019 (COVID-19) vaccine in hematological malignancies (HMs) patients, especially for those who were unresponsive or poor responders to the first two vaccine doses. Therefore, we analyzed humoral response after booster dose in HM patients, focusing on disease- and treatment-specific effects.
Methods: We prospectively evaluated antibody response after the booster dose in 422 HM patients and 80 healthy controls (HCs) using the following schedule: at 14-90 days after the second dose (post-D2), at least 150 days after the second dose if available (pre-D3), and at 14-90 days after the booster dose (post-D3). Patients with evidence of prior COVID-19 were excluded. Antibodies against the SARS-CoV-2 spike protein receptor-binding domain (anti-S) were quantified using the Elecsys Anti-SARS-CoV-2S assay (Elecsys Anti-SARS-CoV-2 ECLIA, Roche Diagnostics, Burgess Hill, UK) performed on a Cobas 8000 e801 (Roche Diagnostics), with a level of ≥0.8 U/mL considered positive.
Higher anti-S levels correlate with reduced risks of symptomatic COVID-19, with ≥264 binding antibody units/mL providing a vaccine efficacy of 80% against symptomatic infection with the alpha variant (Feng et al., Nat Med 27, 2021). Therefore, we defined serological status at post-D2 as follows: anti-S ≥264 U/mL as "responder,” 0.8 U/mL to 264 U/mL as "low-responder,” and <0.8 U/mL as "seronegative."
Results: Among 422 patients (median age, 75 years; 226 men and 196 women), 199 (47.2%) had lymphoid neoplasms (LNs), 133 (31.5%) had plasma cell dyscrasia (PCD), and 90 (21.3%) had myeloid neoplasms (MNs). All patients and HCs received three doses of an mRNA-based vaccine (BNT162b2 or mRNA-1273). At post-D2, the median anti-S level was 204.5 (interquartile range [IQR] 12.9-648.2) U/mL, which significantly declined at pre-D3 (median 91.7 [IQR 6.3-36.8] U/mL, P<0.001). The booster dose markedly improved antibody responses with approximately 100 times higher median anti-S levels (8834 [IQR 753.2-22653.7] U/mL, P<0.001) than pre-D3, although the response was significantly lower than that in the HC group (median 23371.5 [IQR 17144.5-41131.7] U/mL, P<0.001).
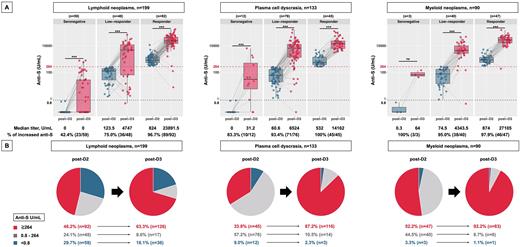
Next, we evaluated anti-S levels according to the three disease groups (LNs, PCD, and MNs) to assess the heterogeneous effect of the disease on humoral response (Figure 1). Across all groups, almost all responders at post-D2 had markedly elevated anti-S levels at post-D3 (LN, median 23891.5 [IQR 14479.7-43904.7] U/mL; PCD, 14162 [IQR 6199-22942] U/mL; MN, 27105 [IQR 16655-42941] U/mL). However, among seronegative and low responders at post-D2, the LN group was less likely to have elevated anti-S levels (seronegative, 42.4%; low responders, 75.0%) at post-D3 than the PCD (83.3% and 93.4%, respectively) or MN groups (100% and 95.0%, respectively). Consequently, the LN group had the lowest probability of achieving anti-S levels ≥264 U/mL at post-D3 (LN, 63.3%; PCD, 87.2%; MN, 92.2%). Regarding seronegative patients, a larger fraction of LN patients (36/59, 61.0%) remained seronegative than that of PCD (3/12, 25%) and MN (1/3, 33.3%) patients.
We also evaluated treatment-specific effects (within 6 months before the booster dose) on humoral response. Patients receiving the anti-CD20 antibody had extremely blunted antibody responses at post-D3 (median anti-S, 0 [IQR 0-0] U/mL), with an increase in anti-S levels in only 3/32 (9.4%) patients. In the PCD group, patients receiving proteasome inhibitor had significantly higher post-D3 anti-S levels than those receiving anti-CD38 antibody (median 9716 [IQR 2609.2-19878] U/mL vs. 3244 [IQR 559.5-9619] U/mL, P=0.007) but not among those receiving immunomodulatory drugs (vs. 3670 [IQR 761.7-17509.2] U/mL, P=0.105). Patients on Janus kinase inhibitor had improved antibody responses at post-D3 (median 2286.5 [IQR 490.2-3554.2] U/mL) despite significantly low rates of responders at post-D2 (only 10%).
Conclusions: Booster vaccination largely improved humoral response across patients with various types of HMs and treatments. However, a subset of patients, especially those in the LN group who recently received the anti-CD20 antibody, had inferior antibody responses, highlighting the need to develop more refined strategies to protect these vulnerable populations.
Disclosures
Takamatsu:SRL: Consultancy; Bristol-Myers Squibb: Honoraria; Sanofi: Honoraria; Ono: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal